📖7th Grade Chapter 5 Blog: Solids, liquids and gases.
5.1 What Makes an Object a Solid, Liquid, or Gas?
Have you ever wondered why ice is hard but water flows? The answer lies in how matter is arranged.
Solids are hard and keep their shape because their particles are tightly packed in a fixed, regular pattern.
Liquids flow and take the shape of their container because their particles are close together but can slide past one another.
Gases spread out and fill any container because their particles are far apart and move freely.
👉 Try this: Look around you and identify one solid, one liquid, and one gas!
5.2 The Particle Theory of Matter
The Particle Theory States that All matter is made of tiny particles that are in constant, random motion. The way these particles are arranged and how much energy they have determines the state of matter. Let’s break it down:
🔍 Solids:
Particles are very close together, arranged in a fixed, repeating pattern.
They vibrate in place but can’t move freely.
This makes solids rigid and incompressible.
🔍 Liquids:
Particles are close together but not in a fixed pattern.
They slide past each other, allowing liquids to flow and take the shape of their container.
Liquids have a fixed volume but no fixed shape.
🔍 Gases:
Particles are far apart and move quickly in all directions.
They fill any space, have no fixed volume, and can be compressed.
👉 Fun Fact: This is why a balloon expands when you blow air into it!
What is🧪 Brownian Motion?
Robert Brown (1773–1858) noticed that tiny particles (like pollen in water) move in a random, jittery way. This movement called Brownian Motion is caused by collisions with invisible water molecules, proving that matter is made of particles that are always moving.
5.3 Changing States of Matter
Matter can change from one state to another. These are called physical changes, and they’re reversible.
Melting: Solid to liquid
Freezing: Liquid to solid
Boiling: Liquid to gas
Condensation: Gas to liquid
Sublimation: Solid to gas (like dry ice)
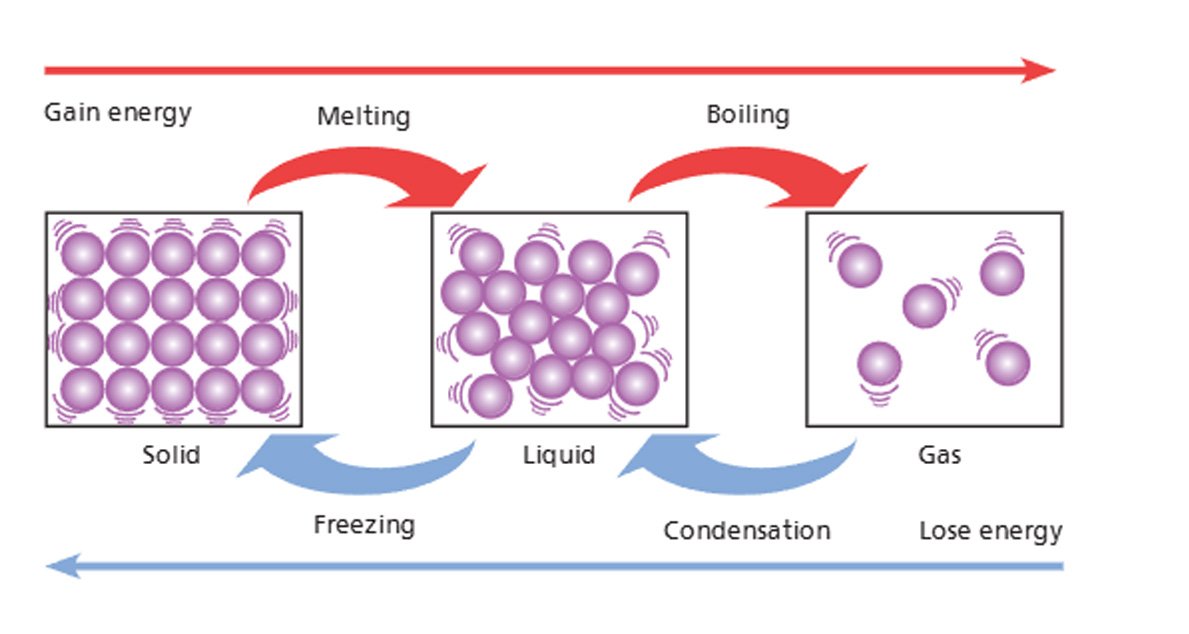
5.4 The Particle Theory and Changes of State
When matter is heated, particles move faster and can change state
Figure: 1.2 Changes of states of matter
🔥 Melting: When a solid such as ice is heated, its particles absorb heat energy and begin to vibrate more rapidly. As they gain sufficient energy, they overcome the strong forces holding them in a fixed arrangement and begin to move more freely, resulting in a change from a solid to a liquid. This process is called melting, and for ice it occurs at 0ºC.
💨 Boiling: If heating continues, the liquid water particles gain even more energy and move faster. When they have enough energy to break free completely from the attractive forces holding them together, they escape from the liquid and become a gas. This process is known as boiling, and it occurs at 100ºC for water.
💧 Condensation: When a gas is cooled, its particles lose energy and move closer together. As a result, the gas changes back into a liquid. For example, water droplets form on the outside of a cold glass due to condensation.
❄️ Freezing: As shown with the blue line. As a liquid cools, its particles lose energy, slow down, and arrange in a fixed pattern, becoming a solid. This process is known as freezing, and for water it occurs at 0ºC
🌬️ Evaporation:
Similar to boiling liquid particles at the surface of a liquid can gain enough energy to escape into the air as gas, even below boiling point. This process is called evaporation and it happens slowly at all temperatures. For example, water in the ocean evaporates under the sun’s heat, even though the ocean itself does not reach the boiling point. Evaporation is part of the water cycle.
The water cycle is the continuous movement of water on, above, and below the surface of the Earth. Although the total amount of water on Earth remains constant, it changes state as it moves through different parts of the cycle.
One of the most important processes in the water cycle is evaporation, where water from the surface of oceans, rivers, and lakes gains energy from the sun’s heat and changes from a liquid to a gas (water vapor). This water vapor rises into the atmosphere.
As the water vapor cools in the atmosphere, it condenses into tiny water droplets, forming clouds. This process is known as condensation.
When the water droplets in clouds become large and heavy enough, they fall back to Earth as precipitation rain, snow, or hail. This water can collect in rivers, lakes, and oceans, or it can infiltrate into the ground, replenishing groundwater supplies.
From these water sources, evaporation occurs again, continuing the endless cycle of water movement and state changes from liquid to gas and back to liquid or solid ensuring that water is always moving and available for living things on Earth.
🧊 Sublimation:
Sublimation is the process by which a substance changes directly from a solid to a gas without first becoming a liquid. This occurs when the particles at the surface of the solid gain enough energy to overcome the forces holding them together and escape as gas.
Solid particles gain enough energy to become gas without becoming liquid.
Examples of substances that undergo sublimation include dry ice (solid carbon dioxide) and iodine crystals.
💡 Conclusion:
Compare the rates at different temperatures.
Does higher temperature increase the rate of evaporation?
Why might this be the case?
🌎 Real World Connection:
Evaporation is key in the water cycle, helping water move from oceans to clouds and back to Earth as rain. Understanding evaporation helps us learn about climate and weather!
⚡ Bonus Topic: Plasma; The Fourth State of Matter
Plasma is like a gas but with electrically charged particles. It glows and conducts electricity! Examples include lightning, neon lights, and even the sun!